Microdevice for blood component separation from whole human blood
Blood is considered as the human health repository due to the presence of various components that act as a biomarker and indicator. Human health information is easily available through blood component testing as each element exhibits different information about human health. The blood component separation is a crucial step to perform the blood component testing for disease diagnosis. Several diseases require an early diagnosis to increase the survival rate of patients. The traditional approach of centrifugation is tedious, time-consuming, and requires skilled and trained personnel. Apart from this limitation, the integration of a centrifuge with a point-of-care device is virtually not possible. Here, we develop a passive microfluidic device to address this complex issue in a more effective manner.
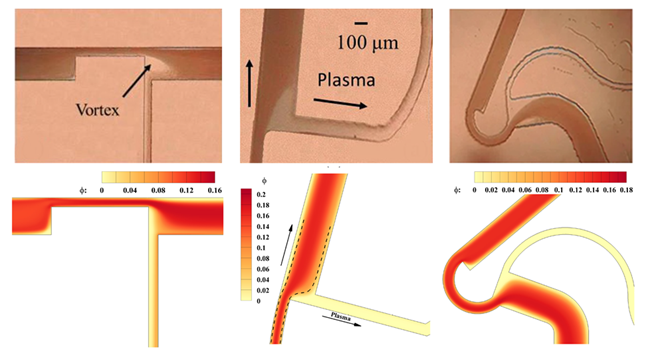
We have further developed a novel blood-based passive microdevice for blood plasma separation and extraction of platelet-rich plasma and platelet-poor plasma from whole human blood. The performance of the in-house developed microdevices is extremely interesting and encouraging. The blood plasma separation (BPS) achieved a separation efficiency of 100% for whole human blood, while the platelet-rich plasma (PRP) microdevice attained ~15-fold enrichment. We are conducting extensive experiments to develop a new microdevice for WBC separation, and so far, we have achieved up to about 8-fold WBC enrichment. Our developed microdevices require minimal blood samples and chip area, utilize simple fabrication techniques, and are hemolysis-free and clog-free devices. We are developing an integrated novel single microdevice to separate all blood components from whole human blood.
Developing the above blood-based passive microdevices requires many blood-based experiments to optimize the dimension, geometry, and other governing parameters, especially during the initial phase of microdevice development. To address the challenges posed by conducting multiple blood-based experiments, we have developed a continuum-based two-fluid numerical model to simulate blood flow in microchannels and microdevices. This numerical framework has been extensively tested and shows an excellent agreement with experimental results. It serves as a valuable tool for developing and optimizing future microdevices, during the early stage of their development. Additionally, we are currently working on integrating other blood components, such as platelets and white blood cells, into the framework to further enhance its utility for optimizing dimension, geometry, and governing parameters in blood-based passive microdevices.